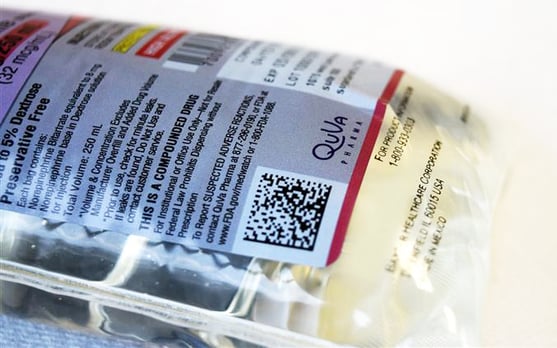
2D Data Matrix Barcodes
Powered by GS1 StandardsQuVa Pharma continues to demonstrate leadership in 503B by now offering 2D data matrix barcodes to its product labeling — a significant enhancement for our customers and increased safety for their patients.
The addition of 2D data matrix barcodes will support traceability, interoperability, and streamline workflows for hospitals and health systems, as well as help support improved inventory management through greater transparency and information exchange.
Supercharge your supply chain with 2D data matrix barcodes:
- Drive visibility for products throughout the supply chain and inside the hospital
- Complement your DSCSA compliance requirements for pharmaceutical manufactured products with the addition of QuVa's 503B labeled products
- Enhance safety in dispensing and administration
- Reduce manual data inputs
- Gain efficiencies in inventory management
- The 2D data matrix barcode will be applied to both case packaging labels and unit of use primary labels on all QuVa products
- The barcode will include product number, lot number, expiration date, and serialization
QuVa's products with 2D barcodes will start releasing in May 2023 and continue throughout the year across the breadth of QuVa's compounded sterile product portfolio.
Contact Us

“QuVa embraces solutions for transparency, information collection, and data exchange that can enhance patient care. We understand hospitals’ desire for operational efficiencies and achieving compliance with FDA’s Drug Supply Chain Security Act (DSCSA). Interoperability across hospitals track-and-trace workflows, and even inventory management and medication dispensing platforms, can be enhanced by QuVa applying 2D barcodes. Even though DSCSA compliance is not a requirement for 503B products, our investments to add GS1 2D data matrix barcodes to all product labels was compelling. We are please to be doing our part to support a more transparent and accountable supply chain that enables hospitals to more accurately track medications, reduce errors and improve patient safety."
Stuart Hinchen, QuVa Pharma Co-founder and CEO
