Innovative New Label Improvements
Developed to reduce medication errors and improve patient safety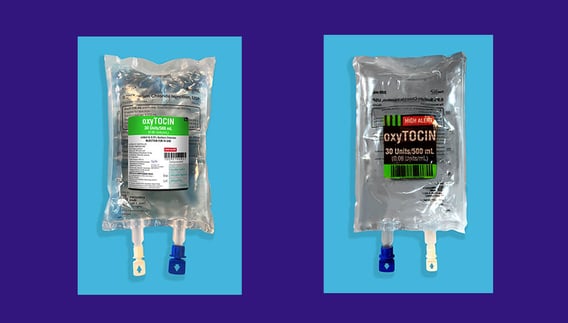
New Two-Sided IV Bag Labels Developed to Raise Patient Safety and Reduce the Risk of Drug and Dosing Errors
- Two-sided label makes important product information visible from both sides of the IV bag
- Enables clear visual inspection of the product given backside of IV bag is not obstructed with a label
- Developed in consultation with customers to meet ISMP’s 2022 best practice recommendations
- We will continue to release our enhanced two-sided labels onto all QuVa bags throughout 2024
-
25% of all medication errors are attributed to name confusion, and 33% to packaging and/or label confusion.1
-
The FDA receives over 10,000 reports every year associated with medication errors. Their #2 recommendation to reduce errors is proper labeling.2
End-to-end, we NEVER sacrifice quality
Contact us if you would like to learn more about our New Two-Sided IV Bag Labels.
Contact Us
1Berman, A. Reducing Medication Errors Through Naming, Labeling, and Packaging. Journal of Medical Systems. 2004; Issue 28, P9 - 29.
2Working to Reduce Medication Errors. U.S. Food & Drug Administration. 2019.

“Delivering an innovative, two-sided, single IV bag label applied to the frontside of the IV bag that contains all required drug and dosing information, while also containing key visual cues and critical product information that is clearly seen when looking through the IV bag solution from the backside, is an advancement in quality aimed at reducing drug and dosing errors. The two-sided label provides customers the ability to easily visually inspect the quality of the product inside the bag, which is not possible when simply applying two standard labels, one on each side of the bag. QuVa Pharma is committed to provide high quality product enhancements that improve safety, and this is another example of our development track record that will help our hospital partners meet their clinical needs.”
Stuart Hinchen, CEO QuVa Pharma.
